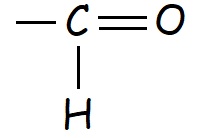
Organic Chemistry
Aldehyde Lab
General Structure of Aldehydes:
In chemistry, an "alde" is an organic compound containing the functional group represented below:
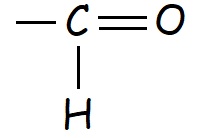
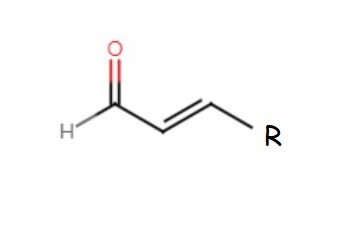
This functional group, by itself, is known as an aldehyde or formyl group. The generic formula for an aldehyde, including the alkyl chain is shown below:
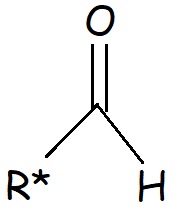
The "R" represents the "rest of the alkyl chain". The aldehyde group is somewhat polar. This makes compounds composed of aldehydes slightly soluble in water. Generally, the larger the aldehyde molecule, the less soluble the molecule will be.
Important Aldehydes:
The following compounds are aldehydes that you might be familiar with:
1. Methanal (commonly called formaldehyde): used as a preserving fluid for biological organisms for many years and is still used as one of the main embalming fluid chemicals.
2. Ethanal (commonly called acetaldehyde): chemical that occurs naturally in coffee, bread, and ripe fruit. Also produced by the partial oxidation of ethanol by the liver enzyme alcoholdehydrogenase and is the contributing cause of the "hangover effect" after one consumes too much alcohol.
3. Cinnamaldehyde: naturally occurs in the bark of cinnamon trees and is the chemical that gives cinnamon its distinctive flavor and odor.
4. Vanillin: naturally occurs as the primary component of the extract of the vanilla bean. Vanillin is used as a flavoring agent in foods, beverages, and pharmaceuticals.
5. Citral: an aldehyde found in the peel of the lemon fruit (along with a variety of other citrus fruits and tropical plants). Citral has an oily consistency that is highly fragrant and is used in various aroma based products, as well as a food additive to enhance the flavor experience of certain recipes.
Today's activity will concentrate on the 3 aldehydes that can be safely consumed.
After completing this aldehyde activity, you will be able to:
Identify and draw the structural formula of the select aldehydes.
Recognize the odor and taste of the pure (or unrefined) form of the select aldehydes.
Recognize the odor and taste associated with the practical use for each select aldehyde.
Cinnamaldehyde:
Structure
Cinnamaldehyde is an organic compound that belongs to a group of compounds known as "phenylpropanoids". It is a pale, yellow, viscous liquid that occurs naturally in the bark of the cinnamon tree as well as other species of the genus Cinnamomum. A phenylpropanoid consists of a phenyl group (which is just a benzene ring connected to a 3-carbon aklyl chain (the propanoid structure).
| Phenyl Group | Propanoid Structure |
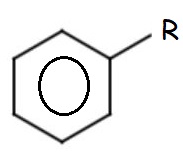 |
 |
The cinnamaldehyde structure, when the two groups are connected, looks like this:
The accepted IUPAC name for cinnamaldehyde is: 3-phenyl-2-propenal
Applications:
Cinnamaldehyde is used as a flavoring agent in chewing gum, ice cream, candy, and beverages. It has also been used as an aromatic in perfumes and candles. It is also a safe and effective mosquito larvae insecticide. Interestingly, ground up beechnut husk aromatized with cinnamaldehyde is marketed and sold as powdered cinnamon. So in many cases "cinnamon" may not actually be cinnamon from the cinnamon tree. The only part from the actual cinnamon tree is the chemical added to the beechnut husk powder. Some cinnamon, though, is real......you just have to read the label carefully.
Odors and Flavors of Cinnamaldehyde:
You have three samples of materials that contain cinnamaldehyde. Test and describe the odors and flavors of each and record your reactions/responses on your lab data sheet.
Vanillin
Structure:
Vanillin is an organic compound that is classified as a phenolic aldehyde. The functional groups that make up a phenolic aldehyde are as follows:
| Aldehyde | Hydroxyl Group | Ether | Phenol Group |
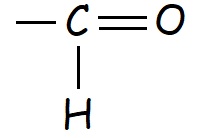 |
 |
|
 |
The complete vanillin structure looks like this:
The IUPAC name for vanillin is: 4-hydroxy-3-methoxybenzaldehyde
Scientists tinkering with the ether portion of the vanillin molecule discovered that by adding an additional methyl group to the existing methyl group on the ether portion of the molecule produced a more potent and aromatic derivative of vanillin. This new (and improved ?) version of the vanillin molecule is called ethylvanillin.
The complete ethylvanillin structure looks like this:
The IUPAC name for ethylvanillin is: 3-ethoxy-4-hydroxybenzaldehyde
Applications:
Vanillin is extracted from the vanilla bean or synthetically produced. Natural vanillin is more expensive than the synthetic version of vanillin so many foods, beverages, and perfumes have used the synthetic vanillin as way to cut costs. The synthetic ethylvanillin is about 3 or 4 times more potent as vanillin or its synthetic counterpart. Ethylvanillin is more expensive than real vanillin and therefore reserved for the more expensive products. Chocolates produced with ethylvanillin have sold for more than $1000 and perfumes with ethylvanillin may be as expensive as $2500 per ounce!
Odors and Flavors of Vanillin
You have three samples of materials that contain vanillin. Test and describe the odors and flavors of each and record your reactions/responses on your lab data sheet.
Citral
Citral is naturally occuring aldehyde found in the oils of the following plants:
Lemon myrtle
Litsea cubeba
Lemon grass
Lemon tea-tree
Clove basil
Lemon verbena
Citral is also located in the oil of the peelings of the following fruits:
lemons
limes
oranges
The molecular structure of the citral aldehyde is:
The correct IUPAC name for citral is: 3,7-dimethyl-2,6-octadienal
Applications:
The citral aldehyde has a strong lemon (or citrus) scent and can be used as an aroma compound in perfumes as well as in essential oils. Citral is also one of the main components of citronella which can be used as a mosquito repellant. This is often accomplished by burning "citronella candles or torches" infused with the chemicals. The odors produced will supposedly keep mosquitoes away.
The citral in the peels of lemons, limes, and oranges can be used as an ingredient in cooking foods. This is done by grating the peeling into thin shavings which is then added to the recipe. This is referred to as "zest", as in "lemon zest" or "orange zest". The odor of the citral is supposed to enhance the flavor experience of the dish.
Essential oils containing citral are sold as therapeutics for antioxidants, antimicrobials, anticancers, and antidiabetics. Results are mixed as the accuracy and usefulness of these claims as these reports are merely anecdotal with no real clinical trials conducted.
Odors and Flavors of Citral
You have three samples of materials that contain citral. Test and describe the odors and flavors of each and record your reactions/responses on your lab data sheet.
Complete the Conclusion Questions section of you lab data sheet.